[INTERVIEW] SD Biosensor's having a great pandemic
![Lee Hyo-keun, CEO of SD Biosensor, Korea's largest test kit maker. [SD BIOSENSOR]](https://koreajoongangdaily.joins.com/data/photo/2022/01/16/9a744f32-9224-4ce7-b7a4-68879021daff.jpg)
Lee Hyo-keun, CEO of SD Biosensor, Korea's largest test kit maker. [SD BIOSENSOR]
SD Biosensor has achieved many firsts in the area of Covid-19 test kits.
Its Standard Q test kit was the world’s first antigen test kit to receive emergency use authorization from the World Health Organization (WHO) in September 2020. It was also the first antigen test kit that Korea’s Ministry of Food and Drug Safety granted formal use approval.
Before Covid-19, SD Biosensor was a front runner in diagnostic kits for previous outbreaks such as Ebola in 2014 and Middle East respiratory syndrome in 2015.
“SD Biosensor's technical skills [in the diagnostic field] and the way we see the market are our two main strengths,” SD Biosensor CEO Lee Hyo-keun, told the Korea JoongAng Daily. “As soon as the Covid-19 pandemic emerged, we were faster than anyone to realize the importance of antigen test kits, and as a result, we were able to sell more Covid-19 antigen kits than any other company.”
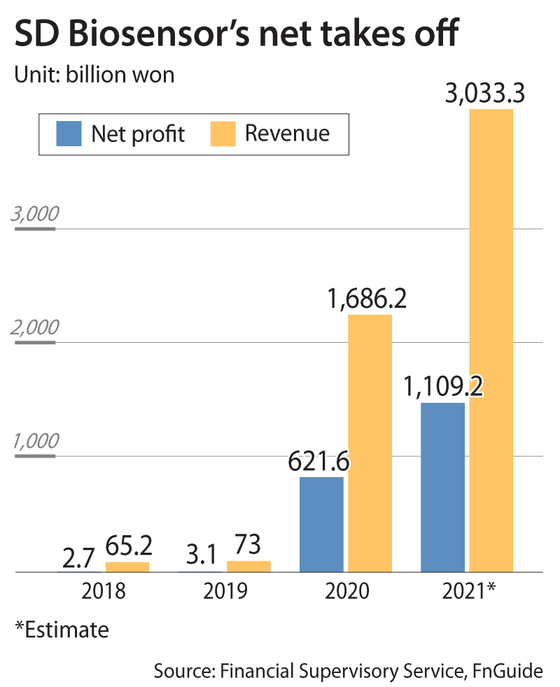
As of the end of October, the Suwon, Gyeonggi-based company had sold a total of 900 million Covid-19 antigen test kits and 25 million polymerase chain reaction (PCR) kits in some 120 countries — and sales have only increased since.
SD Biosensor went public on the Kospi in July, making it Korea’s largest test-kit developer by market capitalization. It is also No. 1 in terms of sales.
Below are edited excerpts of Lee’s interview with the Korea JoongAng Daily.
Q. It took only six weeks for SD Biosensor to develop Covid-19 test kits after the genetic sequencing of the coronavirus was shared. Did you realize the pandemic would be so huge?
A. It’s very difficult to predict the outbreak of infectious diseases that occur in new forms. But SD Biosensor spares no expense in researching and developing new test kits as an effort to prepare for a new pandemic. In 2020, when we acquired prequalification from WHO for our test kits for malaria and human immunodeficiency virus (HIV), we upgraded our production capacity to the maximum. Coincidentally, Covid-19 emerged, and we were able to use our expanded capacity to manufacture Covid test kits.
SD Biosensor has achieved many firsts. How has it done that?
Rapid test kits is an area in which we have considerable expertise. We own core technology and facilities that can develop kits more rapidly than any competitors in the market. It takes quite a long time to develop antigen test kits, but we could be the front runner, especially in the field of antigen kits, based on our long research and accumulated know-how.
Such success was impossible without all our employees in different fields, including researchers who have expertise in diagnostics, staff at manufacturing sites and people in marketing and sales who worked hard to promote our products globally.
Now, we are set to enter the global market with a new product, a point-of-care testing (POCT) product [which is also known as near-patient testing, referring to a test using a device or test kit in the presence of the patient and without the need to send a sample to a laboratory.]
In your 11 years, annual revenues have grown more than 100 times and you went public in July. What attracted investors?
Definitely SD Biosensor’s technical skills [in the diagnostic field] and the way we see the market. Since our establishment, we’ve always tried hard to develop technologies that can be applied to all diagnostic products including rapid antigen tests and fluorescent immunodiagnostic systems. I could confidently say that SD Biosensor has the largest business portfolio of products compared to any other Korean in-vitro diagnostic companies.
Technology determines the future of a company. SD Biosensor is currently recruiting more researchers in order to enhance our technologies even further more.
As soon as the Covid-19 pandemic emerged, SD Biosensor was faster than anyone to realize the importance of antigen test kits, and as a result, we were able to sell more than anyone. I believe SD’s way of viewing the market was appreciated by investors.
Some critics say rapid antigen testing always fall behind PCR testing in terms of sensitivity and accuracy. What are your thoughts on this?
The main difference between the two tests is how they diagnose diseases. PCR testing use sequences in the genetic material present in samples and can be used to diagnose a patient, either by their presence or absence. In the case of antigen testing, it relies on the specific binding of an antigen [a protein displayed on the surface of the virus] in the sample to antibodies immobilized in the spots where the indicator lines appear.
Making the explanation simpler, it is quite similar to a pregnancy test. Most people use pregnancy test kits to know if they are pregnant or not and visit an obstetrics and gynecology for confirmation. Having both kits’ in the right places will help the country prevent the virus from further spread.
In addition, SD Biosensor’s antigen test kits achieves 90 percent in sensitivity and 96 percent in specificity while our PCR testing achieves 100 percent in both. They are being used in countries all over the world.
Some say that test kit companies' prospects will diminish as more vaccines and treatments are used globally.
In order to prescribe treatment and confirm the efficacy of vaccines, diagnosis is necessary. The development of treatments and vaccines will drive the growth of the test kit market.
As of 2020, about 90 percent of your sales came from Covid-related products. What will you do after the Covid pandemic ends?
Last year, we launched Standard M10, a POCT system that enables simple, fast and accurate diagnosis of infectious disease, drug resistance and genetic testing. POCT may sound quite unfamiliar to the public, but it is the next-generation way of diagnosing diseases with smaller samples. POCT kits have taken all the good points of rapid antigen test kits and PCR testing, so they can produce accurate results within one hour, which will be very useful for patients that need emergency preventive measures.
Standard M10 received a lot of attention from many countries even before its release. The product, which has already been used abroad, is one of our future growth engines. SD Biosensor is planning to develop products not only for Covid, but also for HIV, hepatitis B virus, and multidrug-resistant tuberculosis.
SD Biosensor owns around 150 diagnostic products that can detect various types of diseases other than Covid. We are set to export more to international health organizations, and if so, we can see big profit from them.
Also, the company is planning mergers and acquisitions [M&As] and investments in a bid to strengthen our business portfolio in the long term.
![An SD Biosensor employee demonstrates the company's self-test kit in its facility in Suwon, Gyeonggi. [YONHAP]](https://koreajoongangdaily.joins.com/data/photo/2022/01/16/bc18f459-0095-4146-8695-bb455abdd346.jpg)
An SD Biosensor employee demonstrates the company's self-test kit in its facility in Suwon, Gyeonggi. [YONHAP]
SD Biosensor has been making aggressive investment in in-vitro diagnostic companies including Russia’s Eco Diagnostica and Suwon-based UXN. What’s your goal?
SD Biosensor is preparing to enter the U.S. market with our quality rapid antigen kits and POCT products. Of course, we are very positive about M&As that can contribute to strengthening our competitive edge, though we can’t specify the details at the moment.
Our ultimate goal is to become the No. 1 company in global in-vitro diagnostic market.
It seems like Covid-19 brought a lot of changes to the biopharmaceutical industry. How are you going to respond to them?
The pandemic educated the public about businesses of diagnostic companies. When people hear about diagnosis, they will think of self-test kits, or PCR testing that they get at testing sites. But the diagnostic area is way broader than they realize. SD Biosensor will look for further growth by developing a range of products in the fast-growing diagnostic market.
BY SARAH CHEA [chea.sarah@joongang.co.kr]










with the Korea JoongAng Daily
To write comments, please log in to one of the accounts.
Standards Board Policy (0/250자)