ExoRenal aims to give kidney dialysis patients their lives back
-

- LEE JAE-LIM
- lee.jaelim@joongang.co.kr
![ExoRenal CEO Jake Lee speaks during an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]](https://koreajoongangdaily.joins.com/data/photo/2024/10/05/d9b31040-8fee-432a-bc19-e4fa8c6d5191.jpg)
ExoRenal CEO Jake Lee speaks during an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]
[GAME CHANGER]
Allowing patients with chronic kidney disease to receive dialysis when and where they prefer might sound unimaginable.
But that is what ExoRenal founder Jake Lee envisions in 10 years.
Lee believes that his vision might be realized for some 600 million chronic kidney disease patients worldwide. On average, these patients undergo hemodialysis three times a week, with each session lasting three to five hours. When you factor in the time spent preparing and traveling to and from the medical facility, patients can easily dedicate more than six hours of their day to treatment.
Headquartered in Baltimore, Maryland, ExoRenal’s two devices are in the process of receiving U.S. Food and Drug Administration (FDA) approval.
Lee has some specific goals ahead for the treatment industry — to have hemodialysis as a service customized to the patient’s daily life, making it easier not only for the patient themselves but for their families and caregivers as well.
“In the United States, attending your child’s graduation is considered a great honor,” Lee said in a recent interview with the Korea JoongAng Daily at ExoRenal’s research lab in Seoul. “Let’s say my child graduates from a good school in San Francisco while I live in New Jersey. But if I’m a chronic patient, there may be a good possibility that I can’t attend the graduation because my dialysis schedule cannot be adjusted.
“What we envisioned — though it might still sound far-fetched to some — is to have the treatment be tailored to the patient’s needs. Nowadays, users don't go to utilize services. Services come to the clients. We have food and other commodities delivered to our very doorsteps from e-commerce places like Coupang. I think dialysis could work the same way with a subscription system in place.”
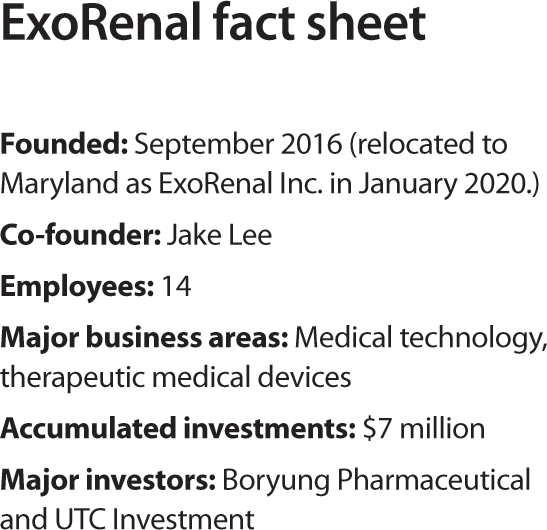
ExoRenal, since its founding in 2016, attracted a total of $7 million from Seoul-based Boryung Pharmaceutical and venture capital UTC Investment. The startup’s headquarters operates businesses related to investment, finances and FDA approval, while it also has a separate lab known as “P&A” in Seoul for domestic licensing and research and development (R&D).
The following interview has been edited for length and clarity.
![ExoRenal's two flagship devices for portable hemodialysis, XRO and Xkidney, displayed at the CES 2024 in Las Vegas in January.[EXORENAL]](https://koreajoongangdaily.joins.com/data/photo/2024/10/05/29a708c1-4c44-4f7a-a3b1-be0d257465af.jpg)
ExoRenal's two flagship devices for portable hemodialysis, XRO and Xkidney, displayed at the CES 2024 in Las Vegas in January.[EXORENAL]
Q. What are your two flagship products that are under development?
A. We currently have a portfolio of two products, both undergoing FDA approval. xKidney is an ultra-compact hemodialysis device, and XRO is a portable water treatment system for hemodialysis.
Xkidney is our flagship product, designed not only to replace the current hemodialysis machines used in hospitals but also to eventually support easy home dialysis for patients. It is the smallest and lightest dialysis device for chronic kidney disease in the world, yet it achieves dialysis efficiency comparable to larger machines. Additionally, it supports treatments like continuous renal replacement therapy (CRRT) for chronic kidney disease patients. The device integrates various functions, minimizing user operation and maximizing convenience.
XRO is capable of replacing hospital water treatment systems for hemodialysis. It can be connected to existing hospital equipment to enable dialysis and, in the future, when home dialysis becomes more common, it will work together with Xkidney to allow easy dialysis at home, hotels or other locations.
XRO recently began safety and efficacy testing for FDA approval. Although the timeline may fluctuate a little depending on our Series A investment schedule, we plan to submit the FDA approval application within this year. Meanwhile, the final prototype of Xkidney is currently in development. Major design specifications have been finalized, and testing and efficacy validation for FDA approval will begin after the product showcase at the American Society of Nephrology (ASN) in late October. We aim to complete FDA approval for Xkidney by the third quarter of next year.
![ExoRenal CEO Jake Lee poses for a photo after an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]](https://koreajoongangdaily.joins.com/data/photo/2024/10/05/464d87d4-52e2-44bc-98db-7f89c4ea956e.jpg)
ExoRenal CEO Jake Lee poses for a photo after an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]
By size, how much has it become portable?
What I wanted to create was a dialysis device that an adult woman could carry around.
xKidney is one-sixth of its original size. Both devices [xKidney and XRO] weigh approximately 23 kilograms each. But they cannot reduce the time span of the treatment itself. But their biggest advantage is that if the era does come when home dialysis is possible and patients can receive treatment every day instead of three times a week, the machines can carry out the functions of a natural kidney, therefore increasing the survival rate of the patients.
It is also an attractive commodity for enterprises as well because the manufacturing unit price of this ultra-compact hemodialysis device is less than half of what our competitors offer.
So how were you able to achieve this portability? What were the technological hurdles you’ve faced in creating your devices?
Dialysis, as we know, is a treatment that purifies a patient’s blood by circulating it outside the body. A similar example of external blood circulation can be found in artificial hearts.
By applying the heart’s natural pulsatile mechanism, we developed a pump that mimics the heart’s function of moving blood. This allowed us to eliminate three essential components traditionally required in conventional dialysis machines, making them much more lightweight.
One of the major hurdles we faced in developing the machine came from controlling the accuracy of fluid levels during dialysis, which demands extremely high precision in a consistent manner.
Dialysis patients come to the hospital with complete loss of kidney function, meaning that they cannot urinate or pass fluids naturally. When they arrive for treatment, one of the key objectives is to remove excess fluids from their bodies.
During a 240-minute dialysis session, if the fluid levels are off just for a minute, the patient might be okay. Maybe they could even handle [the error] for even 10 minutes, but if it goes on for an hour or two, the patient’s fluid balance can change drastically and, therefore, directly affects their blood pressure.
To sum up, managing the fluid balance of the patient accurately is the core technology in our dialysis devices, and it took us a lot of time to achieve that level of precision consistently. As of now, we’ve succeeded in achieving the target error rate of below 4 percent.
![ExoRenal CEO Jake Lee poses for a photo after an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]](https://koreajoongangdaily.joins.com/data/photo/2024/10/05/4ca21994-9ede-4071-a1de-bf6a46bfb9b5.jpg)
ExoRenal CEO Jake Lee poses for a photo after an interview with the Korea JoongAng Daily in August at the startup's office in Gangseo District, western Seoul. [PARK SANG-MOON]
What is the business model for your devices?
We’re planning to receive FDA approval and launch in the United States first and then in Korea.
For the United States, the selection of components and manufacturing processes are to be finalized in the second half of this year, and we will be able to establish a concrete pricing range. Our company has a phased market entry strategy, initially targeting the hospital dialysis market after product approval, focusing on the business-to-business (B2B) sector. We are planning to collaborate with distributors specializing in dialysis products in specific regions and are in the process of expanding our connections with them.
For Korea, there are no manufacturers of dialysis machines in the country, which makes the regulatory guidelines related to that a gray zone. When I consulted domestic experts, the prevailing opinion was that, since the standards from the Ministry of Food and Drug Safety are unclear, they might request exploratory clinical trials. All in all, we forecast that there will likely be intense internal discussions in order for the machines to be granted approval.
For domestic distribution, we are discussing with Boryung Pharmaceutical, [which has invested 6 billion won in ExoRenal] to commercialize our machines.
If portable dialysis machines are so convenient, how come it’s only now that such devices were invented?
The demand for portable dialysis machines or home dialysis has grown significantly in recent years, but major companies like Medtronic or Johnson & Johnson don’t sell dialysis products. There are several reasons for this, but one of them is that, until recently, the dialysis market was relatively small.
Ironically, after founding the company, the social necessity for portable and home dialysis grew even stronger. The Covid-19 pandemic left a significant impact on high-risk groups like dialysis patients. We witnessed multiple cases where patients couldn’t get timely dialysis treatments due to hospital closures and ended up passing away.
However, the pandemic is just part of the reason for the growing demand for portable home dialysis. Globally, the number of dialysis patients has been skyrocketing, doubling roughly every 10 years. Many countries are struggling with the financial burden that dialysis places on national health insurance systems. The U.S. Medicare program is a prime example, but countries like Korea, Australia, Canada, and many European nations face the same challenge. Shifting hospital-based dialysis to home dialysis could help reduce the financial strain on national health insurance systems.
This financial pressure is well-reflected in the executive order issued by then-U.S. president Donald Trump in the summer of 2019. It aimed to transition more than 60 percent of new dialysis patients to either kidney transplants or home dialysis. Since that executive order, home dialysis has been strongly encouraged in the United States, with hospitals and clinics that promote it receiving substantial incentives.
![An employee at ExoRenal explains about its two flagship devices for portable hemodialysis at the CES 2024 in Las Vegas in January. [EXORENAL]](https://koreajoongangdaily.joins.com/data/photo/2024/10/05/3cb8c522-253e-4f9f-b942-3f4e41ef271b.jpg)
An employee at ExoRenal explains about its two flagship devices for portable hemodialysis at the CES 2024 in Las Vegas in January. [EXORENAL]
You were originally a researcher and professor until you decided to establish your company in the middle of your career. What made you veer into becoming a startup founder?
During my master's program, I studied both mechanical engineering and medical science, which later led me to pursue biomedical engineering for my Ph.D. I completed my doctorate, focusing on developing a new method for hemodialysis. Specifically, I introduced a dialysis method that increased efficiency by incorporating the mechanism through which the heart pumps blood into the dialysis process.
After earning my Ph.D., I worked in marketing at Fresenius, the leading company in this field, where I gained insights into customers and products. I then broadened my research on hemodialysis while working as a research fellow at the University of Michigan and later as a research professor at the University of Vermont.
Through these years of research in dialysis, I witnessed the closed nature of the field firsthand. I began to wonder why companies like Fresenius and Baxter weren't releasing better products. It was around this time that I developed a passion for creating a new hemodialysis device — one that could provide better treatment while also helping reduce the financial burden on national healthcare systems.
Of course, I didn’t consider starting a company from the beginning. It took several years for me, as an engineer, to turn my passion for developing better products into a commitment to founding a business. But in the end, I resolved to start this development on my own.
What are your plans for expansion and attracting investment?
We plan to begin Series A funding at the end of this year and wrap up the process by spring of next year.
Much of the detailed aspects, such as investment amount and valuation, have already been determined, and we have been expanding our connections with domestic and international investors since the beginning of this year. Through Series A funding, we aim to complete the product approvals for xKidney and XRO and begin initial mass production.
Although we have not yet secured any confirmed orders, we receive numerous inquiries from dialysis product distributors in the Middle East, North America and Southeast Asia. Once Series A is completed and FDA approval is within reach in the first half of next year, we plan to actively collaborate with these partners.
BY LEE JAE-LIM [lee.jaelim@joongang.co.kr]










with the Korea JoongAng Daily
To write comments, please log in to one of the accounts.
Standards Board Policy (0/250자)